Introduction
HCOOH, commonly known as formic acid, is the simplest carboxylic acid and has significant applications in various industries. This colorless liquid has a pungent odor and is naturally found in the venom of certain insects like ants and bees. It is widely used in agriculture, textiles, pharmaceuticals, and as a preservative in animal feed. Due to its strong acidic properties and ability to act as a reducing agent, formic acid plays a crucial role in various chemical and industrial processes. In this article, we will explore the properties, production methods, applications, benefits, hazards, and future potential of HCOOH in detail.
What is HCOOH?
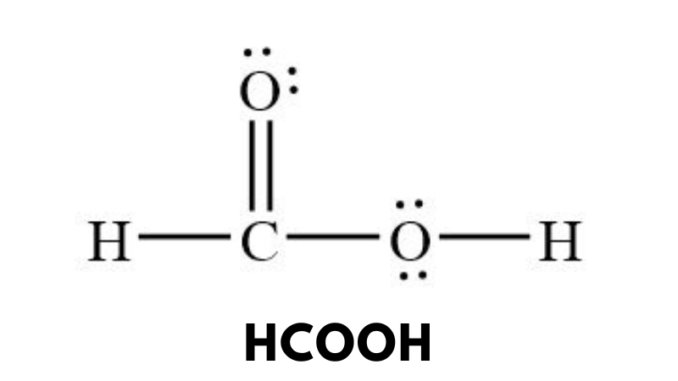
HCOOH, or formic acid, is a chemical compound with the molecular formula HCOOH. It is an organic acid that occurs naturally and is also synthesized for industrial purposes. It was first isolated from ants, which is why it derives its name from the Latin word “formica,” meaning ant. Formic acid is widely used in several industries due to its antibacterial properties, strong acidity, and ability to decompose into carbon dioxide and water under certain conditions. It is also an essential intermediate in organic synthesis and plays a role in various metabolic processes in nature.
Chemical and Physical Properties of HCOOH
Molecular Structure
HCOOH consists of one carboxyl (-COOH) group attached to a hydrogen atom. The structure makes it the simplest member of the carboxylic acid family. It is a polar molecule due to the presence of the hydroxyl (-OH) and carbonyl (C=O) groups, which enable it to form hydrogen bonds with other molecules. These hydrogen bonds significantly influence its physical properties, such as solubility and boiling point.
Physical Properties
- Appearance: Colorless liquid
- Odor: Pungent and irritating
- Boiling Point: 100.8°C
- Melting Point: 8.4°C
- Density: 1.220 g/cm³
- Solubility: Miscible with water, alcohol, and ether
Because formic acid dissolves easily in water and organic solvents, industries transport and use it in various applications. It is also hygroscopic, meaning it can absorb moisture from the air.
Chemical Properties
- Strong acidic nature (pKa = 3.75), making it a strong organic acid
- Acts as a reducing agent, especially in the presence of strong oxidizers
- Forms esters and salts (formates), which have industrial applications
- Decomposes into CO (carbon monoxide) and water when heated to high temperatures
- Can react with alcohols to form esters in the presence of an acid catalyst
Natural Sources of HCOOH
HCOOH occurs naturally in various biological and environmental sources. Some of the major sources include:
- Insect venom: Bees, ants, and wasps use formic acid as a defense mechanism. The acid causes pain and irritation when injected into the skin.
- Plant secretions: Some plants, such as stinging nettles, contain formic acid in their trichomes, which causes a stinging sensation when touched.
- Anaerobic fermentation: Certain bacteria produce formic acid as a metabolic byproduct during the breakdown of organic material. This process occurs in soil, wetlands, and digestive systems of some animals.
- Atmospheric processes naturally form formic acid through oxidation reactions involving volatile organic compounds (VOCs) that plants and industrial processes release.
Industrial Production of HCOOH
Although nature produces formic acid, industries must manufacture it on a large scale to meet demand. The main methods of producing formic acid include:
1. Carbonylation of Methanol
This is the most common industrial method for producing formic acid. It involves reacting carbon monoxide (CO) with methanol (CH3OH) in the presence of catalysts such as sodium methoxide. The reaction produces methyl formate and then hydrolyzes it to yield formic acid.
2. Hydrolysis of Methyl Formate
Methyl formate (HCOOCH3) is hydrolyzed using water and acidic catalysts to produce formic acid and methanol. The process recycles methanol, making this method efficient and cost-effective.
3. Oxidation of Biomass
An environmentally friendly approach to formic acid production involves oxidizing lignocellulosic biomass. This method is gaining traction as industries look for sustainable and renewable sources of formic acid.
Applications of HCOOH
HCOOH has extensive applications across multiple industries due to its versatility. Some of the major applications include:
1. Agriculture and Animal Feed
- Used as a preservative for silage and animal feed to prevent bacterial and fungal growth.
- Helps maintain the nutritional value of feed over extended periods.
- Used as an acidifier in animal nutrition to improve digestion and prevent infections.
2. Textile and Leather Industry
- Used in the dyeing process to help fix colors onto fabric.
- Plays a role in tanning leather by acting as a pH adjuster and removing unwanted materials.
3. Pharmaceutical Industry
- Used as an antibacterial agent in various medicines.
- Commonly found in pain relief creams, lotions, and antiseptic solutions.
4. Chemical Industry
- Acts as an intermediate in the production of various organic compounds.
- Used to manufacture esters, formates, and other chemicals.
5. Fuel Cells
- Formic acid fuel cells (FAFCs) provide a clean and efficient alternative to hydrogen fuel cells.
- Used in portable power generation and low-emission vehicles.
6. Cleaning and Sanitization
- Used as a descaler and disinfectant in industrial and domestic applications.
- Effectively removes mineral deposits and sanitizes surfaces.
Health and Safety Considerations
1. Health Hazards
Formic acid can be harmful if inhaled, ingested, or comes into contact with the skin. Exposure may result in:
- Skin Contact: Severe irritation, burns, and blistering.
- Inhalation: Respiratory distress, coughing, and lung irritation.
- Eye Contact: Pain, redness, and potential blindness.
2. Safety Measures
- Always wear protective gloves, eyewear, and clothing when handling formic acid.
- Ensure proper ventilation in workspaces to prevent inhalation.
- Store in tightly sealed containers to prevent leakage and accidental exposure.
Environmental Impact of HCOOH
HCOOH is considered environmentally friendly due to its rapid biodegradation and low toxicity.
- Breaks down into harmless byproducts like carbon dioxide and water.
- Minimal toxicity to aquatic and terrestrial life compared to stronger acids.
- Used in eco-friendly industrial processes to reduce pollution.
Future Prospects and Innovations
The demand for formic acid will grow as green energy and sustainable industrial applications advance.
- Renewable energy: Increasing use in formic acid fuel cells (FAFCs) for clean energy solutions.
- Biodegradable plastics: Utilized in the synthesis of eco-friendly polymers.
- Advanced pharmaceuticals: Further research into potential medical and antimicrobial applications.
FAQ’s
What is the common name of HCOOH?
HCOOH is commonly known as formic acid.
Is HCOOH dangerous to humans?
Yes, HCOOH can cause skin burns, eye damage, and respiratory irritation upon exposure.
What is the industrial use of HCOOH?
HCOOH is used in agriculture, pharmaceuticals, leather processing, textiles, and chemical synthesis.
Can HCOOH be used as a fuel?
Yes, formic acid fuel cells (FAFCs) are being developed as an alternative energy source.
How is HCOOH produced naturally?
HCOOH is found in insect venom, plant secretions, and microbial fermentation.
See Also: The Most Powerful Doctor in the World
Conclusion
HCOOH, or formic acid, is a valuable organic compound with diverse applications across various industries. From its natural occurrence in insects and plants to its role in sustainable energy solutions, formic acid continues to play a significant role in modern science and industry. While it poses some health risks, proper handling and safety measures ensure its safe and effective use. As industries shift towards green and sustainable practices, formic acid’s potential in renewable energy and eco-friendly applications continues to expand, making it an essential compound for the future.

Leave a Reply